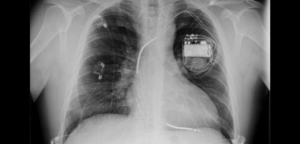
An implantable cardioverter defibrillator (ICD) is an electronic device similar to a pacemaker that is implanted under the skin of a patient.
Cardiac arrest or sudden cardiac death occurs when an abnormal heart rhythm such as ventricular tachycardia (VT) or ventricular fibrillation (VF) disturbs normal heart function and blood circulation to the brain and other important organs. These dangerous arrhythmias without warning can be fatal in a matter of minutes, making them difficult or impossible to treat by conventional methods.
An ICD, sometimes popularly called a defibrillator, is a device, powered by a special battery, which significantly increases survival rate in the event of dangerous (malignant) arrhythmias. ICD cannot prevent arrhythmia occurrence, but it can successfully detect and treat it, restoring normal heart rhythm and save lives.
ICD treats arrhythmias in three ways:
- cardioversion
- defibrillation
- stimulation (anti-tachycardia stimulation)
Cardioversion and defibrillation are both terms that indicate the delivery of an electric shock that interrupts a dangerous arrhythmia and restores a normal heart rhythm. If the patient is conscious the delivery of the shock is painful and is described as a “blow to the chest”. In most cases, however, while in a dangerous arrhythmia, shock is delivered only when the patient loses consciousness and does not feel the shock delivery. Anti-tachycardia stimulation involves the delivery of short cycles of stimulation (5-10). Anti-tachycardia stimulation is not painful, and it is almost imperceptible. Fewer patients may experience an unpleasant feeling of heart palpitations.
Because ICD is a sophisticated computer, it has a memory that records the patient’s heart activity as well as any disturbance of the heart rhythm and their treatment by the ICD. Such monitoring is of great diagnostic value and makes it easier for physicians to treat patients and further adjust the therapy and the device for each patient.
In addition to treating dangerous tachycardia, today’s modern ICD has all the possibilities of a standard pacemaker to treat bradycardia.
An ICD, just like a pacemaker, consists of a pulse generator and one or more electrodes. The pulse generator consists of a special battery that produces electrical shock and drives the entire system, and a computer circuit with an application that monitors the entire system. ICD electrodes monitor electrical impulses from the heart and send information about the patient’s heart activity to the ICD’s computer.
Cardioverter defibrillator implantation. Most often, the surgery is performed under local anaesthesia or deep sedation, and if necessary, it is possible that the patient is completely dormant for the purpose of device testing. The implantation technique is the same as for the implantation of a pacemaker (see: Permanent pacemaker implantation). The implantation lasts about 60 minutes.
Cardioverter defibrillator implantation complications – complications can occur during surgery or after surgery.
Complications during surgery:
- lung collapse (pneumothorax) in 1% of cases – the therapy is to place a drain into thorax
- cardiac perforation (cardiac tamponade) done with an electrode, which causes blood to accumulate in the closed space around the heart; in 1% of cases; therapy is placing a drain in the area where blood is collected (pericardium)
- bleeding at the operative incision where the ICD’s battery (pulse generator) is located; additional risk is seen in patients prescribed with drugs that affect clotting (varfarin, acetylsalicylic acid, etc.)
- the risk of device infection is less than 1%
- electrode displacement in the heart occurs in about 1% of cases and usually requires a new procedure where the electrode/s are repositioned
- death; death while implanting an ICD is less than 1:500.
Complications after:
- implantation area infection – usually the entire system must be removed and re-implanted after the infection has resolved
- electrode dysfunction – electrodes are the most sensitive part of the ICD, and thus strong mechanical forces from the outside can move or break the electrode, requiring new procedure and the electrode replacement
- inadequate detection and delivery of electric shock
- premature ICD battery discharge
Instructions for patients after implanting a cardioverter defibrillator.
After the implantation of the ICD, it is necessary to attend regular check-ups and controls, which are performed every 3-6 months. At the check-up completely painless control of the device is done with a special programmer. The ICD is a device that operates on the principle of wireless technology and that makes it very easy to test and adjust without necessity to open a wound.
If a shock was delivered. If the patient has felt shock delivery from an ICD, he/she should immediately report to the hospital where the device was implanted. There, the doctor will determine why the electric shock occurred and whether additional adjustments to the device or the introduction of additional medications are needed.
Fear of cardioverter defibrillator. Some patients describe the delivery of a shock as very uncomfortable and painful. However, most patients tolerate this discomfort because it saves life. Some patients may feel anxious and depressed about their dependency on the device or they fear of device failure. Talking to a doctor or psychologist can be helpful.
Restriction in driving a vehicle. Following the implantation of the ICD, it is necessary to follow the doctor’s instructions regarding driving. Because VTs and VFs most commonly cause loss of consciousness in patients, it is possible to lose control of the vehicle while driving. Generally the recommendations are:
- you can drive a vehicle 3 months after the ICD was implanted
- it is not recommended to drive for 6 months after ICD shock delivery
In any case, you should consult your cardiologist regarding operating a vehicle.
Avoiding electromagnetic interference. As ICD’s function depends on adequate recording of heart electrical activity, it is important to avoid strong electromagnetic fields that could interfere with the cardioverter defibrillator.
- use your mobile phone on the opposite side of the ICD
- household appliances (TV, radio, toaster, microwave) can be used normally as long as they function correctly
- electromagnetic security systems at the shop entrances, courtrooms, airports and other high-security areas – it is recommended not to stay in the passage or vicinity of similar devices – you should pass through them at normal speed and avoid standing near them
- medical examinations such as X-rays, computerized tomography (CT) and/or ultrasound do not interfere with the ICD’s function
- magnetic resonance imaging (MR) may interfere with the ICD’s function and you should consult your cardiologist or a nurse
- lithotripsy which is used in the kidney stones treatment and transcutaneous electroneurostimulation (TENS) as a pain management method may interfere with the ICD’s work and you should consult your cardiologist or a nurse
- patients are required to inform all medical staff (doctors, dentists, nurses, engineers) that they are cardioverter-defibrillator carriers in order to carefully plan out medical procedures
Pregnancy. Women with implanted cardioverter defibrillator can normally carry out a pregnancy without increased risk to the fetus. If there are cardiac or other diseases in the medical history, it is necessary to consult your cardiologist about possible risks, possible medication changes, and care plans during pregnancy.


 Aritmije KBCSM
Aritmije KBCSM